Stainless steels do not have a single universal melting point; depending on grade and chemistry their melting behaviour falls within approximately 1,325–1,530 °C (2,417–2,786 °F), with common commercial grades clustering between 1,375 °C and 1,455 °C. For example, austenitic 304 typically has a solidus ≈ 1,400 °C and a liquidus ≈ 1,450–1,455 °C, while some ferritic and martensitic grades (e.g., 410) melt nearer the upper end of the range (≈ 1,480–1,530 °C). These ranges are driven by alloying (Ni, Cr, Mo, C, etc.), and by whether one quotes a single melting temperature or a melting range (solidus → liquidus).
What “melting point” means for alloys: solidus vs liquidus
For a pure element there is a single sharp melting temperature. For multi-component alloys — such as stainless steels — melting normally occurs over a range. Metallurgists use two terms:
-
Solidus: the temperature at which the alloy just begins to melt (first appearance of liquid).
-
Liquidus: the temperature at which the alloy becomes fully liquid (last solid disappears).
When specifications list “melting point” for stainless steel they usually mean the practical melting range between solidus and liquidus (or they provide approximate mid-range values). For many common stainless alloys the solidus is about 1,375–1,450 °C and the liquidus falls about 30–80 °C higher depending on composition.
Typical melting ranges by stainless-steel family
Short summary first, then a table below:
-
Austenitic (300 series, 200 series): typically the lowest melting ranges among commercial stainless grades because higher nickel content generally depresses the solidus/liquidus slightly. Example: 304 ≈ 1,400–1,455 °C; 316 ≈ 1,375–1,400 °C (varies by source and exact chemistry).
-
Ferritic (400 series): higher chromium with little/no nickel; melting ranges often higher, e.g., 430 sometimes quoted 1,425–1,510 °C.
-
Martensitic (410, 420, 440): often near the upper range, e.g., 410 ≈ 1,480–1,530 °C.
-
Duplex (2205 and similar): intermediate; melting range can sit between austenitic and ferritic values (e.g., 1,385–1,445 °C for some duplex).
-
Precipitation-hardening (17-4PH, 15-5PH): often around 1,400–1,440 °C depending on the alloy variant.
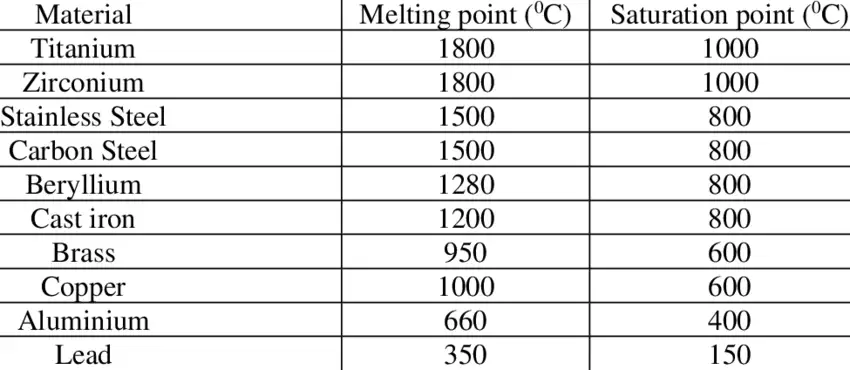
Grade-by-grade quick reference table
Note: values are typical published ranges (solidus → liquidus). When specifying heat-treatment or casting, always use supplier/heat-lot data or an authoritative materials datasheet for the exact alloy.
| Grade / Designation | Typical solidus (°C) | Typical liquidus (°C) | Typical Fahrenheit |
|---|---|---|---|
| 304 / 304L (Austenitic) | 1,400 | 1,450–1,455 | 2,550 → 2,650 °F |
| 316 / 316L (Austenitic) | 1,375 | 1,400 | 2,507 → 2,552 °F |
| 2205 (Duplex) | 1,385 | 1,445 | ~2,525 → 2,633 °F |
| 430 (Ferritic) | 1,425 | 1,510 | 2,597 → 2,750 °F |
| 410 (Martensitic) | 1,480 | 1,530 | 2,696 → 2,786 °F |
| 420 / 440C (Cutlery, high-C martensitic) | 1,450 | 1,510 | 2,642 → 2,750 °F |
(Table synthesis derived from standardized datasheets and association data; small discrepancies arise between sources because of allowable compositional windows and measurement technique.)
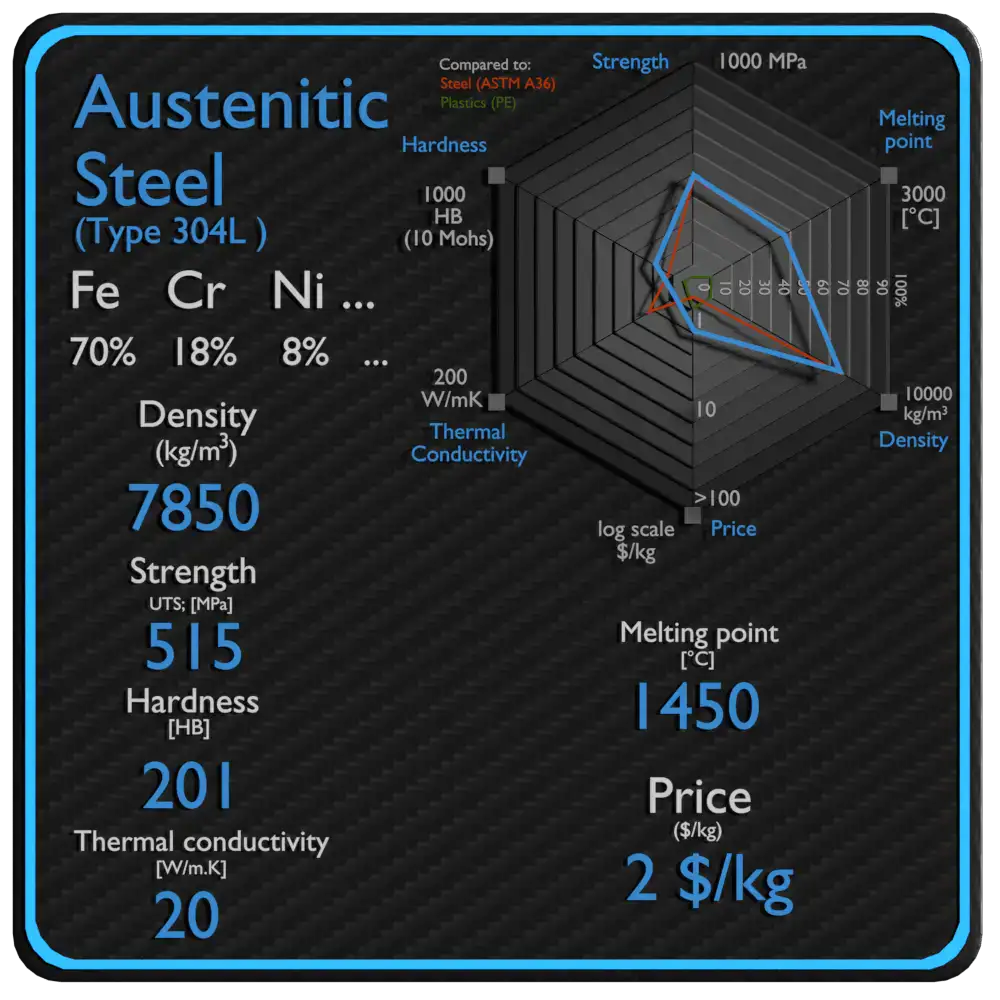
How alloying elements shift melting behaviour
A stainless alloy’s melting range is not random — it’s controlled by chemistry. Key influences:
-
Nickel (Ni): tends to stabilize the austenitic phase and generally lowers the melting range slightly versus Fe–Cr compositions; high-Ni alloys (austenitics) commonly melt at a modestly lower temperature than high-Cr, low-Ni ferritics.
-
Chromium (Cr): chromium has a high melting point itself (≈ 1,863 °C), and higher chromium fractions can raise parts of the phase diagram, widening melting ranges and shifting liquidus upward.
-
Molybdenum (Mo): added for pitting resistance and high-temperature strength; Mo modifies liquidus/solidus locally and can widen the melting window.
-
Carbon (C): small carbon additions form carbides, which affect local melting behaviour (e.g., low-melting eutectics around carbides or sulfides) and may change apparent melting range in metallurgical testing.
-
Minor elements / contaminants (S, P, Si, Mn): sulfur and phosphorus can form low-melting constituents (sulfides, phosphides), which reduce local fusion temperatures and create hot-short behavior or weldability issues.
A practical takeaway: two “304” heats from different mills may show slightly different melting-range endpoints because of permitted chemistry bands (e.g., Ni can vary ±1–2% and C varies). For precise casting or additive-manufacturing work use the melt-shop analysis for that heat.
Phase diagrams, eutectics and why ranges exist
Alloy melting behaviour is best understood on multi-component phase diagrams. Stainless steels are based on Fe–Cr–Ni (and other minor elements). Interactions generate eutectic or peritectic reactions in some compositional zones. Where a eutectic exists the alloy may show a relatively sharp melting reaction; elsewhere partial melting begins earlier and finishes later, producing a wide liquidus gap.
In simple terms:
-
If the alloy composition lies near a simple binary eutectic, melting can begin at a lower temperature.
-
If it lies in a complex multi-phase field, the solidus may be lower and the liquidus higher — a broader melting interval.
Manufacturers use phase diagrams and thermochemical calculations (thermodynamic software like CALPHAD) to predict these behaviours when designing alloys or setting casting parameters.
How melting temperature is measured and reported
Common laboratory techniques:
-
Differential Scanning Calorimetry (DSC) — measures heat flow and identifies endothermic melting events. Good for small samples and precise solidus/liquidus detection.
-
Differential Thermal Analysis (DTA) — similar principle; records temperature differences against a reference.
-
Microscopy after controlled heating — heat samples to stepped temperatures, cool, and metallographically inspect for the first appearance of liquid.
-
Thermocouple measurements in melting rigs — used in foundries for bulk melts (less precise but pragmatic).
Datasheets usually report solidus and liquidus or give a nominal melting range. When quoting numbers for engineering, prefer solidus/liquidus from recognized datasheets (MatWeb/ASM or supplier certificates) rather than single “melting point” values.
Practical implications for fabrication, joining and casting
-
Welding: fusion welding intentionally melts a small volume; knowledge of the melting range guides heat input, filler selection, and cooling rates. Because stainless steels melt over a range, weld pool control and dilution with filler metal matter for avoiding low-melting constituents (e.g., sulphur-rich inclusions).
-
Brazing vs soldering: brazing uses filler metals that melt below the host steel’s solidus and relies on capillary action. Because stainless melts at very high temperatures, brazing alloys must be chosen to melt significantly lower than the stainless solidus.
-
Casting and remelting: in foundries and electric-arc furnaces, awareness of the melting window and the liquidus aids in controlling slag reactions, alloy additions, and preventing loss of volatile elements. Stainless scrap mixes must be managed so final chemistry falls into target bands.
-
Additive manufacturing (AM): laser powder-bed melting and directed energy processes work by melting powder particles. Powder chemistry, particle size, and thermal profile must respect the material’s melting interval to avoid lack-of-fusion or excessive evaporation of alloying elements (especially Ni and Mo). Use powder-supplier datasheets and qualified process windows.
Melting versus service temperature: why service limits are much lower
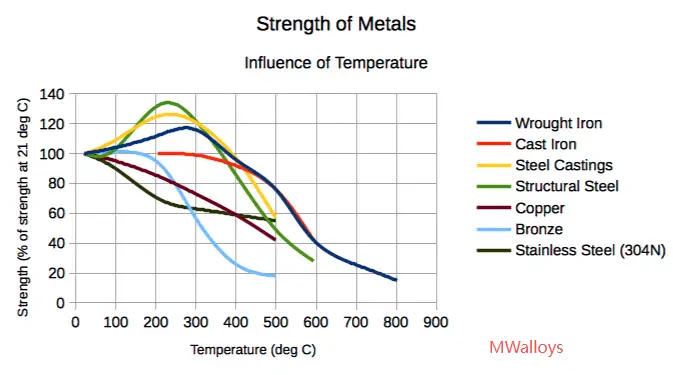
Melting is an absolute end-of-life temperature for structural integrity; however, service or maximum operating temperatures are set far below melting for several reasons:
-
Creep and oxidation occur at far lower temperatures (e.g., many austenitics lose usable mechanical strength well below 1,000 °C). Nickel-rich alloys may keep strength to higher temperatures but still have limits for long times.
-
Phase transformations (sensitization, sigma-phase precipitation) that harm corrosion resistance occur in the 400–900 °C window and are important long before melting.
-
Protective scales break down or sulphidation/oxidation accelerate at intermediate temperatures. That is why maximum recommended continuous service temperatures are often quoted between 600–1,050 °C depending on grade, not near the melting range.
Recycling, scrap melting and industrial furnace practice
In industrial practice, stainless steel is frequently produced in electric-arc or induction furnaces using high proportions of scrap. Key points:
-
Control of chemistry at meltshop is critical; scrap composition varies. Meltshops monitor and add alloying elements to meet grade specs.
-
Inclusion control and slag management matter because low-melting inclusions can reduce effective melting temperature in localized regions, leading to casting defects.
-
Energy considerations: stainless melting consumes significant energy given the high temperatures; process efficiency, oxygen lancing, and scrap preparation affect costs and emissions. WorldStainless and industry reports document meltshop production and energy tradeoffs.
Safety, fluxes and contamination issues when melting stainless steels
Melting stainless steel produces fumes and potentially hazardous oxides (e.g., Cr oxides). Safety controls include:
-
Local exhaust ventilation, fume capture and filtration.
-
Monitoring of alloy loss (evaporation of Cr, Ni, Mo) at high temperatures.
-
Using appropriate refractory linings and fluxes to control slag chemistry and prevent contamination.
When welding, keep in mind that intermetallic or low-melting eutectic phases may form if contamination (e.g., sulfur) is present; these can weaken joints at elevated temperatures.
Standards, data sources and best practice for engineers
For engineering use, rely on authoritative datasheets and standards, such as:
-
Manufacturer material certificates and supplier datasheets (matriculation for the specific heat or batch).
-
Recognized databases like ASM/MatWeb for typical solidus/liquidus values.
-
Industry associations (World Stainless, BSSA, Nickel Institute) for broad overviews and technical bulletins.
Frequently asked questions (FAQs)
Q1. What is the single melting point of stainless steel?
There is no single melting point for “stainless steel” in general. Use the solidus–liquidus range for the specific grade; typical overall industry ranges are ≈1,325–1,530 °C.
Q2. Which common grade melts the lowest?
Austenitic grades (high-Ni compositions) such as some 316 variants may sit at the lower end (≈ 1,375–1,400 °C) relative to some ferritic/martensitic grades.
Q3. Why do published melting numbers differ between datasheets?
Different labs use different test methods (DSC, DTA, larger-scale melting trials) and alloy chemistry tolerances differ between suppliers; reporting can show slightly different solidus/liquidus endpoints.
Q4. Does the melting point affect weldability?
Indirectly. Melting range affects how the weld pool behaves and what filler metals are appropriate; but weldability is also dominated by carbon, sulfur, and phase transformations rather than absolute melting temperature alone.
Q5. Can I melt stainless steel in a propane forge?
Practical bulk melting of stainless requires higher temperatures and better insulation than common propane forges; small-scale melting of thin sections may be possible, but industrial melting is done in induction or arc furnaces.
Q6. Does stainless steel boil or evaporate at melt temperatures?
At typical melting temperatures, volatile losses (especially of nickel and manganese) can occur when molten metal is exposed, but boiling (bulk vaporization) happens only at much higher temperatures and under different circumstances. Proper furnace atmosphere and fluxes control evaporation.
Q7. How does microstructure affect apparent melting?
Pre-existing phases (carbides, intermetallics) and segregations melt locally at different temperatures, so microsegregation widens the effective melting interval and can create early-melting pockets.
Q8. Which is more important: melting point or service temperature?
Service temperature is usually far more relevant for component life. Melting point is an absolute limit; long-term mechanical and corrosion performance is dictated by much lower temperatures.
Q9. Where do I find exact melting data for a heat?
Ask the mill/supplier for the chemical analysis and the specific datasheet for that heat; if needed, request DSC/DTA testing from a certified lab.
Q10. How should I plan welding/brazing given the melting range?
Choose filler metals with appropriate melting intervals, control dilution, and follow qualified welding procedures. For brazing, ensure brazing alloy melts well below the base metal solidus to avoid base-metal melting.
Authoritative references
- ASM / MatWeb — AISI Type 304 stainless steel technical data (solidus, liquidus, melting range)
- WorldStainless — Introduction to stainless steels (industry overview and facts)
- Nickel Institute — Technical bulletin: Austenitic chromium-nickel stainless steels (properties and temperature effects)
- British Stainless Steel Association (BSSA) — Melting temperature ranges for common stainless grades

