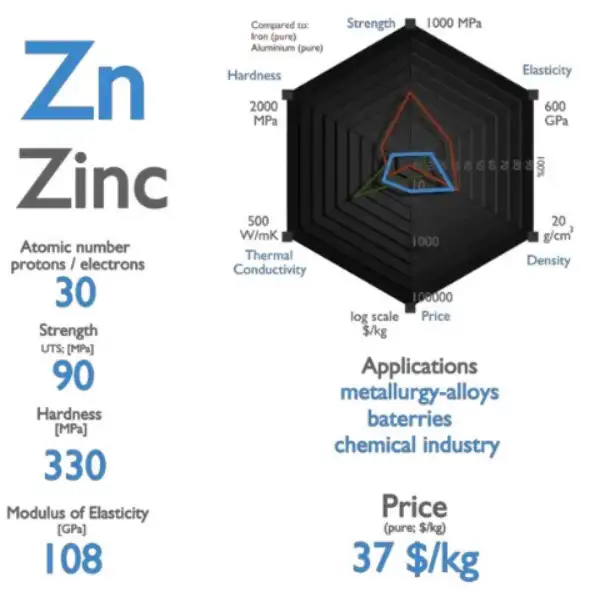
Pure zinc metal has a room-temperature density of approximately 7.13–7.14 g·cm⁻³ (≈7,134 kg·m⁻³) in its common solid form (hexagonal/tetragonal structures depending on allotrope). This value falls consistently in modern reference compilations and is the practical figure used for engineering mass–volume calculations, cost estimations and casting design.
What density means for a metal
Density is the mass per unit volume of a material (commonly expressed in grams per cubic centimetre, g·cm⁻³, or kilograms per cubic metre, kg·m⁻³). For engineers and metallurgists the operational importance of density includes: converting weight to volume for shipping and material costing, estimating sink/float behavior in process fluids, calculating inertia and specific stiffness for structural design, and predicting solidification shrinkage in casting operations. The following sections ground those use-cases with concrete values and working tables.
Standard reference values for zinc (solid & liquid)
-
Common solid value (near 20 °C): 7.134 g·cm⁻³ (often reported as 7.13–7.14 g·cm⁻³ in modern sources). This is the accepted engineering number used in datasheets and elemental property tables.
-
Liquid (at the melting point): when zinc melts (m.p. ≈ 419.5 °C), its density drops — commonly reported values for the liquid at m.p. are in the neighborhood of ≈6.57 g·cm⁻³ (liquid density at m.p. depends on measurement source and exact temperature referenced).
Engineers often round to 7.14 g·cm⁻³ (≈7,140 kg·m⁻³) for quick calculations; authoritative compilations list 7.133–7.14 depending on the edition and measurement temperature.
Temperature and phase dependence
Density is temperature-dependent because the metal’s volume expands with heat. For zinc:
-
Solid-phase density near ambient is the baseline (~7.13 g·cm⁻³ at 20 °C).
-
As temperature increases, thermal expansion causes a gradual reduction in density until the melting transformation (note: crystalline allotropes and phase transitions create non-linear behavior near transformation temperatures). High-quality compilations and some metallurgical handbooks report tabulated densities versus temperature and the coefficients needed for interpolation.
-
The liquid phase at the melting point shows a further drop to values roughly in the mid-6 g·cm⁻³ range (≈6.5–6.9 g·cm⁻³) depending on the source and exact temperature. For casting mass estimates, the liquid density at the pouring temperature is the correct input.
Crystal structure, phases and microstructure effects
Zinc exhibits different structural behavior at low temperatures (there is an α↔β transformation at very low temperatures) and its normal ambient crystal packing affects the packing fraction and therefore the density. Grain size, porosity and cold work will not change the atomic density but will change the apparent bulk density (void fraction) for powders or porous castings. When specifying density for an engineered component, clarify if you mean:
-
Theoretical (crystallographic) density — calculated from lattice parameters and atomic weight;
-
Apparent bulk density — includes voids/porosity and surface roughness;
-
Archimedes (liquid displacement) density — practical method used in the lab for solids.
How alloying and impurities change zinc’s density
Every addition of another element alters the mass-per-volume. Examples:
-
Zinc–aluminum casting alloys (ZA): adding aluminum lowers average density relative to pure zinc (depending on Al fraction).
-
Zinc–copper alloys (brass-like): copper is denser (~8.96 g·cm⁻³), so Cu additions raise the alloy density.
-
Trace contaminants (lead, iron, tin) shift density modestly but can be significant for high-volume pricing or weight-limited designs.
For procurement/specification, always reference the exact alloy designation (e.g., ZA-8, Zamak-3) rather than “zinc” when mass or volumetric precision matters.
Measurement methods and traceability to standards
Common laboratory and industrial measurement techniques:
-
Hydrostatic (Archimedes) method — widely used for finished parts; gives apparent density including closed porosity only if properly sealed.
-
Gas pycnometry — measures true solid volume excluding closed porosity; useful for powders.
-
X-ray/neutron diffraction lattice-parameter method — computes theoretical density from lattice constants for crystalline metals.
-
Online/ex-situ process density measurement — used for molten metal baths with immersion probes or electromagnetic methods.
Traceability: use certified reference materials and methods referenced to national metrology institutes (NIST, NPL, PTB) for high-accuracy requirements. For elemental density and thermophysical constants, consult authoritative compilations (NIST, CRC, RSC, peer-reviewed metallurgy texts).
Conversion tables and quick-reference charts
Table 1 — Core physical constants for pure zinc (reference conditions)
| Property | Value (typical) | Notes / source |
|---|---|---|
| Density (solid, ≈20 °C) | 7.134 g·cm⁻³ | elemental compilations and material databases. |
| Density (solid, rounded) | 7.14 g·cm⁻³ | common engineering rounding used in datasheets. |
| Density (liquid at m.p.) | ≈6.57 g·cm⁻³ | liquid density at melting point (temperature-dependent). |
| Atomic mass | 65.38 g·mol⁻¹ | standard atomic weight. |
| Melting point | 419.5 °C | useful for casting/solidification calculations. |
Table 2 — Unit conversions (practical)
| Unit | Equivalent for zinc (using 7.134 g·cm⁻³) |
|---|---|
| 1 g·cm⁻³ | 1000 kg·m⁻³ |
| 7.134 g·cm⁻³ | 7,134 kg·m⁻³ |
| 1 m³ of zinc | ~7,134 kg |
| 1 litre (1,000 cm³) | ~7.134 kg |
| 1 cubic inch | 0.0163871 L → mass ≈ 0.1169 oz (avoirdupois) |
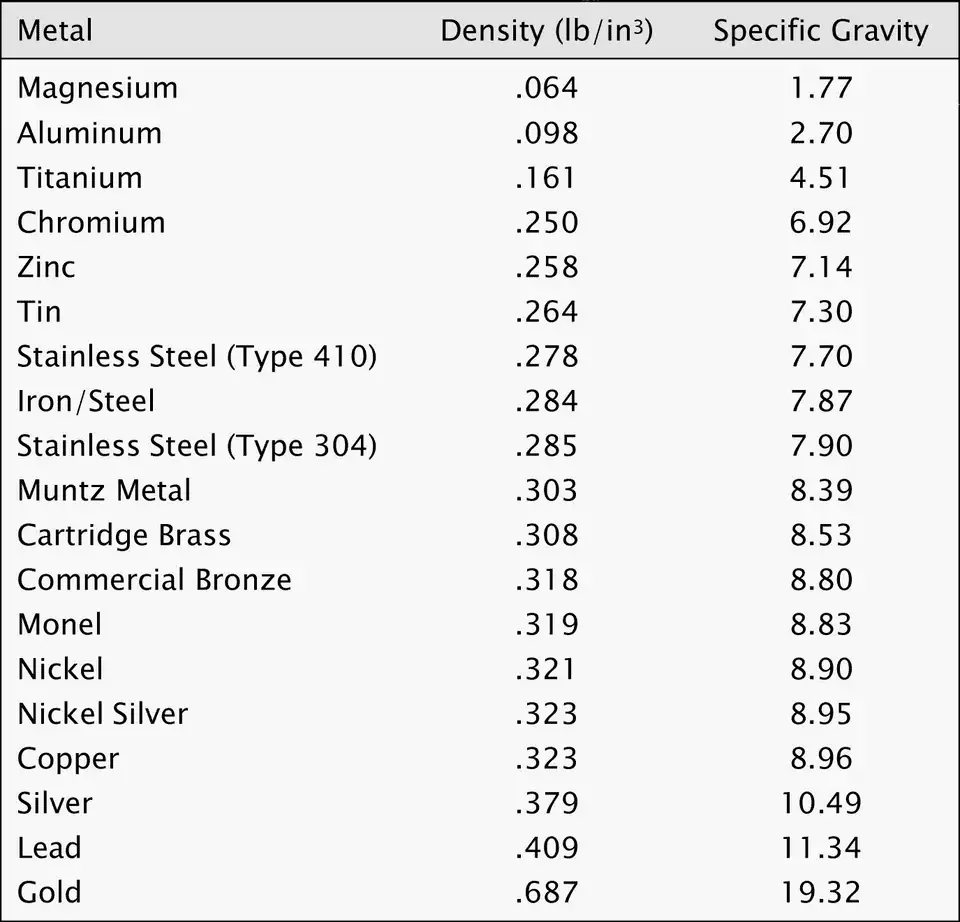
Table 3 — Densities: zinc vs other common metals (quick design context)
| Metal | Density (g·cm⁻³) | Notes |
|---|---|---|
| Aluminum (pure) | 2.70 | much lighter; used when weight is critical |
| Magnesium (pure) | 1.74 | very light structural applications |
| Zinc (pure) | 7.13 | mid-heavy metal; corrosion-resistant coatings |
| Iron / Steel (approx) | 7.85 | denser and stronger in bulk |
| Copper | 8.96 | heavier, excellent conductor |
| Lead | 11.34 | much heavier; used where mass is needed |
Design and manufacturing implications
Galvanizing
Zinc is widely used for hot-dip galvanizing because the density makes it a convenient coating mass per unit area: knowing zinc density lets you convert coating thickness (microns) to mass per area (g·m⁻²) for inventory and cost estimates. Example: a 10 µm coating corresponds to mass ≈ (density × thickness) = 7.134 g·cm⁻³ × 0.001 cm = 0.007134 g·cm⁻² → 71.34 g·m⁻².
Die-casting and shrinkage
Shrinkage allowances in zinc die-casting are calculated from solidification volume changes. Because liquid zinc is significantly less dense than the solid at room temperature, solidification contraction must be accounted for in gating and feeder design to avoid internal voids; use liquid density at pouring temperature and the solid density at ambient to estimate volumetric shrinkage.
Battery anodes and corrosion
Zinc’s density affects how much active mass you can pack into a cell of a given volume. For volumetric energy density calculations, engineers multiply the active mass (kg) by the specific capacity; accurate density values are essential for cell-level design.
Worked examples and templates
Example 1 — mass from volume
You need the mass of a zinc block 200 mm × 150 mm × 50 mm:
-
Volume = 0.2 × 0.15 × 0.05 = 0.0015 m³
-
Mass = density × volume = 7,134 kg·m⁻³ × 0.0015 m³ = 10.701 kg
Example 2 — coating mass from thickness
Stainless panel area 2 m², required zinc coating 20 µm:
-
Volume of coating = area × thickness = 2 m² × 20×10⁻⁶ m = 4×10⁻⁵ m³
-
Mass = 7,134 kg·m⁻³ × 4×10⁻⁵ m³ = 0.28536 kg → 285.36 g total zinc used.
These simple templates are the basis of cost estimation, weight budgeting and process planning.
Common pitfalls when specifying density
-
Not stating temperature — density changes with temperature; always specify the reference temperature (e.g., 20 °C).
-
Confusing “zinc” with zinc alloys — alloys have different densities; always name the alloy standard (Zamak-, ZA-, ASTM grade, etc.).
-
Using bulk vs true density incorrectly — powders, porous castings and foamed parts require different definitions.
-
Ignoring trapped gas/porosity — apparent density can be significantly lower than theoretical values for castings with shrinkage porosity.
Safety and storage notes related to density and phase transitions
Density matters in melt handling: the weight per unit volume of molten zinc and the associated mass of crucibles and ladles dictate hoist capacities and crucible wall stresses. Because molten zinc is less dense than solid at ambient, levelling and feeding systems must be sized to avoid splashing and induce proper feeding during solidification.
FAQs
-
Q: What is the accepted density value for pure zinc used in engineering?
A: Use 7.13–7.14 g·cm⁻³ at ~20 °C for standard calculations; cite the material database or elemental tables in specifications. -
Q: How do I convert zinc density to kg·m⁻³?
A: Multiply g·cm⁻³ by 1000. So 7.134 g·cm⁻³ = 7,134 kg·m⁻³. -
Q: Does zinc density change significantly when molten?
A: Yes — liquid zinc at the melting point is typically in the mid-6 g·cm⁻³ range; the exact value depends on temperature. Use liquid density at pouring temperature for casting calculations. -
Q: Which measurement method gives the most accurate density for solid zinc?
A: For bulk metals, hydrostatic (Archimedes) measurement or lattice-parameter computation (for theoretical density) are standard; gas pycnometry is best for powders. -
Q: How does alloying with aluminum affect zinc’s density?
A: Aluminum reduces average density because Al ≈ 2.70 g·cm⁻³; the alloy’s density scales with composition and phase distribution. -
Q: What density should I use when estimating zinc coating mass for galvanizing?
A: Use the solid density (7.134 g·cm⁻³) and convert thickness to mass per area (mass = density × thickness). Round to appropriate significant figures for cost estimates. -
Q: How to specify zinc density in a technical datasheet?
A: State the value, reference temperature (e.g., “Density: 7.134 g·cm⁻³ at 20 °C”), measurement method and standard reference (NIST/RSC/CRC). -
Q: Does porosity change the density reported on material data sheets?
A: Material datasheets normally show theoretical density for the solid alloy; porous or sintered parts must have apparent or bulk density measured and specified separately. -
Q: For high-temperature processes, how do I adjust density?
A: Use a temperature-dependent expansion coefficient or tabulated density vs temperature data to interpolate the density at the operating temperature. -
Q: Where do I find authoritative density values?
A: National metrology institutes and reputable compilations (NIST PML, RSC periodic table, CRC Handbook, peer-reviewed metallurgy handbooks).
Practical specification language
Density (reference): 7.134 g·cm⁻³ at 20 °C (solid, crystalline zinc). Measurement method: Archimedes method (ISO XYZ / ASTM ABC). Reference: RSC periodic table entry and NIST PML composition data.
Always request batch certificates showing measured density (if critical) and the alloy certificate (chemical composition) to allow calculation of expected density for the supplied lot.
Short checklist for engineers who need to use zinc density
-
State the reference temperature.
-
Name the material precisely (pure zinc or specific alloy designation).
-
Choose correct density type: theoretical vs apparent vs liquid.
-
Use certified measurement methods and request traceable certificates.
-
Apply volume → mass conversions consistently in your BOM and logistics.
Closing practical notes
For most industrial and engineering tasks, using 7.13–7.14 g·cm⁻³ for zinc at ambient temperature delivers reliable mass-to-volume conversions and cost calculations. When working with molten zinc, alloys, powders, or temperature-sensitive processes, specify the temperature and alloy composition and use traceable measurement methods or authoritative tabulations for the correct density value.
Authoritative references
- RSC — Zinc (element fact box and physical constants)
- PubChem — Zinc (element page: physical properties and conversions)
- NIST PML — Composition and reference data for zinc
- MoltenSalt.org — Densities of molten elements (liquid densities at melting points)
- Springer Reference — Zinc: Physical and chemical properties (chapter / handbook entry)

