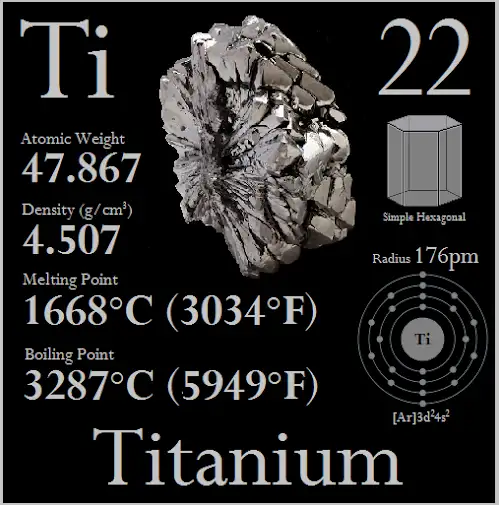
Pure titanium melts at 1,668 °C (3,034 °F; 1,941 K) under standard pressure.
Why the melting point matters
The melting temperature sets a hard upper limit for thermal processing, informs selection of furnaces and melts, and controls which forming or joining methods are feasible for a given titanium product. High-temperature performance, fabrication strategy, and cost all tie back to the element’s melting behavior. For engineering practice, the single-number melting point must be used together with solidus/liquidus ranges for alloys and with phase-transformation temperatures.
Atomic background and crystal phases
Titanium (atomic number 22) belongs to group 4 of the periodic table. At room temperature it takes a hexagonal close-packed lattice (commonly called α phase). When heated above a higher threshold called the beta transus, it transforms into a body-centered cubic β phase. That transformation influences mechanical behavior and melting characteristics because the lattice arrangement, diffusion rates, and solubility of alloying elements differ markedly between α and β. Typical β transus temperatures for commercially pure grades fall near 880–970 °C, while specific alloys may show higher or lower transition points.
Standard value(s) and measurement methods
Most authoritative databases quote titanium’s melting (fusion) temperature at 1,668 °C (1,941 K; 3,034 °F). That number is the consensus from thermochemical reference data and national standards compilations; it is the value used for standard reference tables. Key primary thermochemical measurements and JANAF/NIST compilations underpin this number. Measurement approaches historically include optical pyrometry during high-temperature melting runs, differential thermal analysis, and calorimetric methods; modern studies also use radiance-temperature corrections and careful emissivity calibration to reduce systematic error. The NIST WebBook and peer-reviewed thermophysical studies summarize these determinations.
Solidus, liquidus and alloy behavior
Pure element melting is a single temperature. Practical engineering alloys, however, melt over a temperature interval: solidus (where melting begins) to liquidus (where melting completes). For common aerospace alloy Ti-6Al-4V (Grade 5), solidus values near 1,604 °C (2,920 °F) and liquidus near 1,660 °C (3,020 °F) are typically reported; other alloys and mill conditions produce slightly different ranges. Designers must therefore use alloy-specific solidus/liquidus data rather than the pure-element melting point when specifying casting, welding preheat, or additive manufacturing parameters.
Beta transus and high-temperature transformations
The beta transus marks the α→β transition on heating. Its exact value depends on composition and heat treatment; commercially pure titanium grades often have beta transus near 880–950 °C, while aluminum-stabilized or other alloy systems shift that threshold. The β phase has higher symmetry and different solute behavior, so grain growth rates, recrystallization, and subsequent solidification microstructures depend on whether processing crosses the transus. Proper control of heating/cooling cycles ensures intended microstructures and prevents undesirable coarsening or embrittlement.
Thermophysical properties at and near melting
Key numbers useful for thermal calculations:
-
Melting point (pure Ti): 1,668 °C (1,941 K).
-
Heat of fusion (approx.): ~14.15 kJ·mol⁻¹ (published JANAF/NIST compilations).
-
Liquid density at m.p.: typically ~4.1 g·cm⁻³ (compared with ~4.5 g·cm⁻³ solid at 20 °C). These values matter for casting and shrinkage predictions.
Use these numbers in energy-balance models for melting furnaces, induction-holding calculations, or laser-heat input estimations.
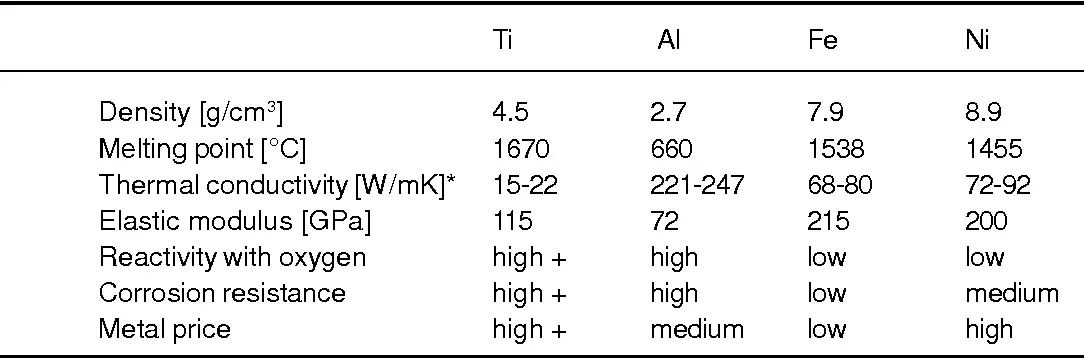
Influence of impurities and alloying on melting behavior
Small amounts of elements such as oxygen, nitrogen, carbon, iron, or aluminum change melting behavior in two ways:
-
Solute lowering/raising of melting range: Certain solutes depress liquidus/solidus while others expand the interval. For example, V and Al shift phase stability, influencing the practical melting window for Ti-6Al-4V.
-
Formation of low-melting eutectics: Contamination by iron or copper may create small-volume eutectic pockets that melt at lower temperature, risking localized melting during thermal cycles. Therefore, feedstock chemistry control and low-oxygen handling are critical during melting and welding.
Practical implication: melting and remelting must be performed in controlled atmospheres and with strict charge chemistry to avoid unwanted low-melting phases.
Industrial melting and refining methods
Because titanium reacts strongly with oxygen, nitrogen, and hydrogen at elevated temperatures, commercial production and remelting adopt protective environments:
-
Vacuum arc remelting (VAR) and electron beam melting (EBM) are widely used to refine and produce ingots with low dissolved gases and controlled composition.
-
Plasma melting and cold hearth melting are also used for segregation control and inclusion removal.
-
Kroll and Hunter processes produce sponge titanium before melting; consolidation then uses vacuum induction melting (VIM) or VAR to make ingots.
Each method affects inclusion population, gas content, and homogeneity — all factors that influence melting behavior in downstream heat treatment and fabrication.
Reference practice: furnaces and melting equipment must include vacuum levels, crucible materials, and shielding regimes designed for titanium chemistry.
Welding, additive manufacturing and melting-related process controls
Welding and laser-based additive manufacturing depend on precise melt pool control:
-
Shielding: Inert gas (argon or helium) or vacuum is mandatory to prevent oxygen/nitrogen pick-up and brittle interstitial-stabilized phases.
-
Heat input: Laser power, beam speed, and layer strategies in powder-bed fusion must be set to produce melt pools that fully fuse powder/track but avoid keyholing or excessive vaporization.
-
Solidification microstructure: Rapid cooling in AM tends to produce fine martensitic α′ in many Ti alloys; post-processing heat treatments can temper and relieve residual stresses.
Solidus/liquidus data for the specific alloy must guide process windows to ensure consistent fusion without excessive evaporation of volatile alloying elements (for example, Al in Ti-6Al-4V).
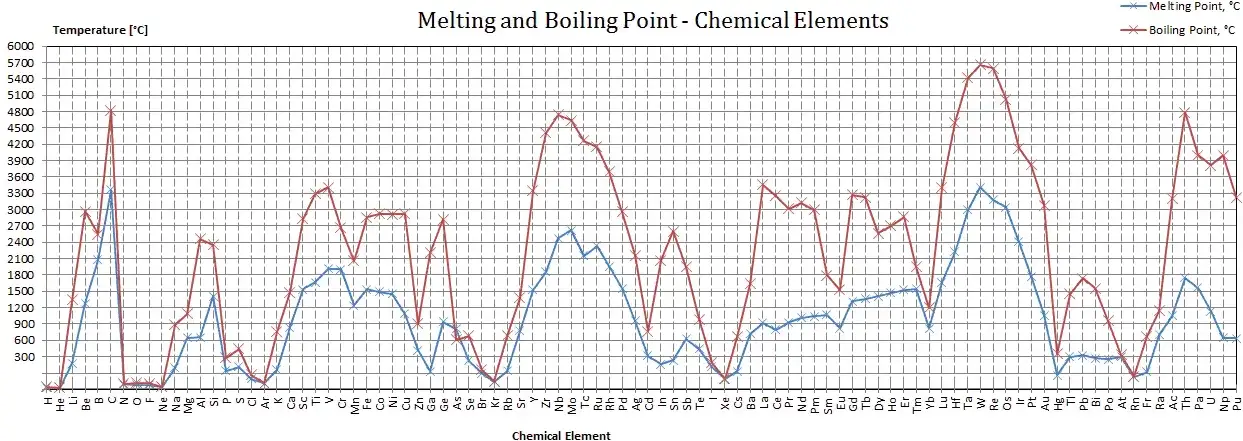
Comparative perspective (table)
Below is a compact comparison of melting temperatures for titanium and several commonly used engineering metals to situate titanium in a materials selection context.
| Metal / Alloy | Typical melting (°C) | Notes |
|---|---|---|
| Titanium (pure) | 1,668 | High melting, lightweight; engineering alloys vary. |
| Stainless steel (304) | ~1,400–1,450 | Lower than Ti but with different high-temp strength. |
| Carbon steel | ~1,420–1,515 | Depends on carbon and alloy content. |
| Nickel (pure) | 1,455 | Close to some steels; nickel superalloys withstand higher service temp due to alloys. |
| Inconel 718 | ~1,300–1,380 (solidus/liquidus vary) | Superalloy, high strength at elevated temperature due to solid-solution/precipitates. |
| Aluminum (pure) | 660 | Much lower; lighter but low high-temp capability. |
Use this table when weighing tradeoffs between weight, high-temperature capability, and cost.
Measurement uncertainty, calibration and best practice
High-temperature melting measurements require:
-
Calibrated pyrometry or contact thermometry tied to fixed-point standards.
-
Emissivity correction when using optical methods; radiant methods must account for wavelength-dependent emissivity changes on molten metal surfaces.
-
Repeatable sample geometry and atmosphere control to avoid surface oxides that alter apparent radiance temperature.
NIST and peer-reviewed thermal-measurement studies discuss radiance-temperature corrections that reduced systematic offsets in reported melting points. For rigorous work, consult JANAF/NIST datasets and recent thermophysical measurement reports.
Practical tables
Table A — Pure titanium key numbers
| Property | Value |
|---|---|
| Melting point (fusion) | 1,668 °C (1,941 K; 3,034 °F) |
| Heat of fusion | ~14.15 kJ·mol⁻¹ |
| Solid density (20 °C) | ~4.50 g·cm⁻³ |
| Liquid density (at m.p.) | ~4.1 g·cm⁻³ |
Table B — Representative alloy solidus/liquidus (typical ranges)
| Alloy | Solidus (°C) | Liquidus (°C) | Source |
|---|---|---|---|
| Ti-6Al-4V (Grade 5) | ~1,604 | ~1,660 | MatWeb / ASM datasheets |
| CP-Ti (Grade 1) | ~1,665–1,670 | ~1,670 | MatWeb datasheets |
| Ti-3Al-2.5V | ~1,700 (max) | — | Material datasheets |
Table C — Common phase temperatures (illustrative)
| Concept | Typical temp (°C) |
|---|---|
| β transus (CP grades) | ~880–950 |
| Typical heat-treatment forging window | 800–1,050 (depending on alloy) |
| Melting / casting window (liquidus region) | >1,600–1,700 |
FAQs
1. What is the melting point of commercially pure titanium?
Pure titanium typically melts at 1,668 °C (3,034 °F). For engineering work, consult alloy-specific solidus/liquidus data.
2. Does Ti-6Al-4V melt at the same temperature as pure titanium?
No. Ti-6Al-4V shows a melting interval: solidus near 1,604 °C and liquidus near 1,660 °C; process windows must use alloy data.
3. How do oxygen and nitrogen contamination change melting?
They do not substantially change the pure-element melting point but cause embrittlement and may promote low-melting intermetallics with impurities. Control atmosphere and feedstock purity to prevent localized melting or weak zones.
4. Which melting method yields the cleanest titanium ingot?
Vacuum arc remelting (VAR) and electron beam melting (EBM) are standard for low-gas, low-inclusion ingots. Cold-hearth melting also helps remove high-density inclusions.
5. Is titanium reactive when molten?
Yes. Molten titanium readily reacts with oxygen, nitrogen, and carbon; melting must occur under vacuum or inert gas to avoid contamination.
6. What is the heat of fusion for titanium and why that matters?
Approximately 14.15 kJ·mol⁻¹. This number feeds into energy calculations for furnace sizing and laser/welding heat balances.
7. Can standard stainless steel furnaces be used to melt titanium?
Only with careful design: refractory linings and oxygen-free atmospheres are required. Plain air-fired or open furnaces are unsuitable.
8. How does the beta transus relate to melting?
The beta transus is far below the melting point but determines high-temperature mechanical behavior and grain structure, which influence casting and welding outcomes.
9. Do additive-manufacturing processes fully melt titanium powders?
Many AM processes produce full melting pools when parameters are correct; powder-bed fusion (laser or electron beam) commonly achieves full fusion but requires control to avoid porosity and evaporation of low-boiling constituents.
10. Where can I find authoritative thermophysical numbers for titanium?
Primary sources include NIST JANAF tables, national laboratory handbooks, and ASM/MatWeb material datasheets. Use these to validate engineering calculations.
What the literature says about measurement precision
High-quality radiance-temperature experiments have produced melting temperatures within a few kelvin of the consensus value; careful emissivity correction and calibration to fixed-point standards reduce scatter. Recent thermophysical measurement reports and compilations (NIST/JANAF; peer-reviewed measurement papers) are the recommended starting point for precision work.
Closing summary
Pure titanium’s melting point at 1,668 °C is a foundational thermophysical datum. For applied engineering, that number must be used alongside alloy solidus/liquidus data, beta-transus temperatures, and thermochemical characteristics (heat of fusion, density change) to set processing windows, select melting equipment, and design joining or additive processes. Use authoritative databases (NIST, ASM/MatWeb, peer-reviewed thermophysical studies) when exact numbers are required for simulations or procurement.
Authoritative references
- NIST Chemistry WebBook — Titanium (thermochemical data & JANAF tables)
- PubChem / NCBI — Titanium element summary (melting point and physical data)
- ASM / MatWeb — Titanium Grade data sheets (typical melting, solidus/liquidus, beta transus)
- NIST Journal of Research — Radiance-temperature measurements and melting-point studies for titanium
- Peer-reviewed thermophysical measurements (open access article on high-accuracy thermophysical properties)

