Steel does not have a single melting temperature; depending on composition and microstructure its solidus–liquidus window typically falls roughly between ≈1,370 °C and ≈1,530 °C (2,500–2,800 °F) for most common carbon and stainless steels. Pure iron melts at about 1,538 °C (2,800 °F), and alloying elements, carbon content and processing history shift the exact temperatures; therefore engineers use melting ranges (solidus to liquidus) rather than a single fixed temperature when designing melting, casting, and welding processes.
What “melting point” means for steels: solidus vs liquidus
Metals that are alloys—like steel—do not usually switch instantly from solid to liquid at one temperature. Instead there is:
-
Solidus — the temperature at which the first liquid appears on heating.
-
Liquidus — the temperature at which the last solid dissolves and the alloy becomes fully liquid.
Between these two temperatures the material exists as a mixture of solid and liquid phases. For many steels the solidus and liquidus are separated by tens to hundreds of degrees depending on composition, which is why practitioners speak of a melting range rather than a single value. The Fe–C (iron–carbon) phase diagram is the fundamental map for understanding these transitions in carbon-containing steels.
Pure iron vs commercial steels — baseline numbers
-
Pure iron: commonly quoted melting point ≈ 1,538 °C (2,800 °F). This is the reference anchor for steels.
-
Carbon steels: typical complete-melt ranges for mild to high-carbon steels fall roughly 1,370–1,540 °C (2,500–2,800 °F); higher carbon tends to broaden and slightly lower the solidus in some compositions while raising the liquidus in others because of complex Fe–C reactions.
-
Stainless steels: depending on grade, stainless steels typically melt in a range close to 1,375–1,510 °C (2,507–2,770 °F); austenitic grades like 304 and 316 commonly have liquidus values in the 1,400–1,450 °C neighborhood but with grade-to-grade spread.
Treat steel melting as a range; use grade-specific datasheets when planning melting, casting or heat-treatment work.
How carbon changes melting behaviour (Fe–C phase relations)
Carbon profoundly alters iron’s phase map and melting behavior. Key points:
-
Carbon lowers the melting temperature of the iron-rich liquid relative to pure iron in certain composition windows and creates eutectic/eutectoid features in the Fe–C diagram. The widely used eutectic (ledeburite) occurs near ~4.3 wt% C at ≈1,147 °C for metastable systems; the eutectoid (pearlite formation) sits at ≈0.76 wt% C and ≈727 °C (a different phenomenon). These features explain why cast irons and hypereutectic alloys solidify differently from steel.
-
For typical steels (0.02–2.0 wt% C), the solidus/liquidus separation grows with carbon and with certain alloying additions; heat flow during melting/solidification is therefore composition-dependent.
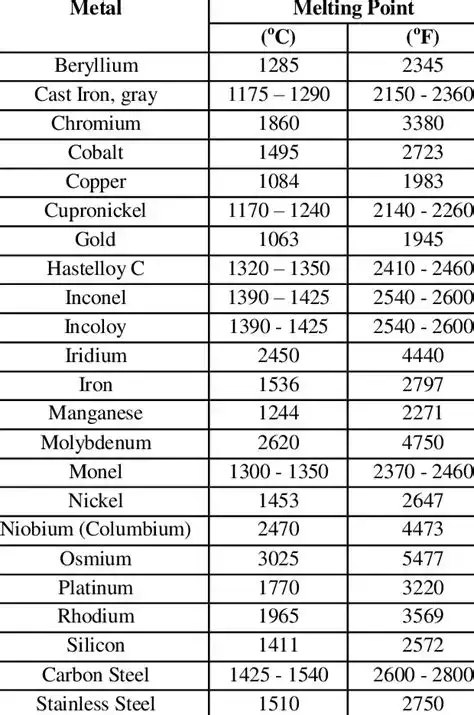
Effect of common alloying elements on melting point
Alloying elements shift solidus and liquidus in different directions and magnitudes. Below is a compact table summarising typical qualitative effects.
Table 1 — Typical influence of alloying elements on steel melting behavior
| Element | Typical role in steel | Effect on melting point (qualitative) |
|---|---|---|
| Carbon (C) | Main hardening interstitial | Alters solidus/liquidus; can widen melting range; complex effect depending on wt% |
| Chromium (Cr) | Corrosion resistance, carbide former | Often raises liquidus slightly; can broaden melting range |
| Nickel (Ni) | Stabilises austenite, improves toughness | Tends to lower solidus/liquidus modestly in many stainless alloys |
| Manganese (Mn) | Strengthening, deoxidiser | Small lowering of melting temp; forms low-melting compounds if excessive |
| Silicon (Si) | Deoxidiser, strength | Slightly lowers melting; helps fluidity in casting |
| Molybdenum (Mo) | High-temp strength | Raises melting behaviour for matrix; forms high-melting carbides |
| Vanadium (V), Titanium (Ti) | Grain refinement, carbide formers | Complex: can form high-melting carbides which locally affect solidus/liquidus |
(Note: quantitative shifts require thermodynamic calculation — use thermochemical databases or CALPHAD for exact predictions.)
Key practical note: combinations of elements are not additive; interactions (e.g., Cr+Ni in stainless steels) can produce non-linear shifts.
Typical melting ranges for common steel families (tables)
Below are practical, engineer-friendly reference tables gathered from metallurgical datasheets and industry compilations (use as guidance; always check supplier datasheets for critical process control).
Table 2 — Representative melting ranges for common steels
| Steel family / Grade | Typical solidus → liquidus (°C) | Typical °F |
|---|---|---|
| Pure iron (Fe) | 1,538 (single point) | 2,800 |
| Low-carbon (mild) steel (≈0.05–0.25% C) | ≈1,420 → 1,470 | 2,588 → 2,678 |
| Medium-carbon steel (≈0.25–0.60% C) | ≈1,430 → 1,490 | 2,606 → 2,714 |
| High-carbon steel (≈0.60–1.2% C) | ≈1,400 → 1,540 | 2,552 → 2,804 |
| Austenitic stainless (304) | ≈1,400 → 1,450 | 2,552 → 2,642 |
| Austenitic stainless (316) | ≈1,375 → 1,400 | 2,507 → 2,552 |
| Ferritic stainless (430 / 410) | ≈1,425 → 1,510 | 2,597 → 2,750 |
| Tool steels (varies widely) | 1,350 → 1,550 (depending on alloy) | 2,462 → 2,822 |
How melting points are measured in the lab
Common laboratory techniques:
-
Differential Scanning Calorimetry (DSC) — measures heat flow and pinpoints phase changes (solidus and liquidus peaks).
-
Differential Thermal Analysis (DTA) — similar principle; records endothermic peaks during melting.
-
High-temperature microscopy — visually records onset of melting.
-
Melting point apparatus and pyrometry — for high-temperature alloys, optical pyrometers and thermocouples in controlled atmospheres are used.
-
Calphad/thermodynamic modelling — predicts phase boundaries from composition, often used to generate solidus/liquidus for complex alloys.
Measurement requires careful atmosphere control (vacuum or inert gas) because oxidation alters observed behaviour; also sample size and heating rate influence apparent melting ranges.
Industrial melting: furnaces and temperature control
Steel is melted and refined in several furnace types; each has temperature/atmosphere implications:
-
Electric Arc Furnaces (EAF) — flexible, high power; used for scrap remelting and alloying control. Ideal for specialty steels and smaller batch melts.
-
Induction furnaces — clean, efficient for smaller heats; good control of temperature and mixing.
-
Basic Oxygen Furnace (BOF) — converts hot metal from blast furnace to steel; operates at very high thermal flux but has different process goals (carbon removal).
-
Cupola — older iron-casting furnace; used largely for cast iron, not modern steelmaking.
Operators aim to heat above the liquidus for casting, but control bath superheat (degrees above liquidus) to regulate fluidity, gas evolution, and solidification kinetics. Refractories, furnace atmosphere and deoxidation practice strongly affect melt cleanliness and apparent melting behaviour.
Welding, brazing, forging — what temperatures matter
For fabrication, the melting range is less important than solid-state transformation temperatures and safe working temperatures:
-
Welding: welding melts local material; effective welding temperatures exceed local liquidus, but the integrity of welds depends on puddle chemistry, dilution, and cooling rate. Avoiding local liquation in heat-affected zones (HAZ) is a key concern.
-
Brazing/soldering: use filler metals with melting points below the steel solidus to join without melting base metal.
-
Forging: hot forging is done well below the melting point but above recrystallisation ranges (e.g., austenitizing range). For steels, forging temperatures typically range from ~900–1,250 °C depending on grade.
Material behaviour close to melting: strength, oxidation, and microstructure
Approaching the melting region steel’s mechanical strength falls rapidly; surface oxidation and scaling accelerate; carbides and other precipitates can dissolve or coarsen. In stainless steels, protective oxide films break down at elevated temperatures; for many applications service temperature limits are set far below melting to preserve mechanical and corrosion properties. BSSA and manufacturer datasheets provide recommended maximum continuous service temperatures distinct from melting temperatures.
Standards, test methods and quality control
Relevant standards and references for testing and specification include:
-
ASTM standards for chemical analysis and melting-related tests (see ASTM standards for steel analysis and ingot testing).
-
ISO standards covering steel classification and testing.
-
ASM Handbook and technical chapters cover melting, furnaces and heat treatment processes. Practical metallurgical labs use calibrated thermocouples, inert atmospheres, and standard reference materials (SRMs) when reporting melting/solidus/liquidus data.
Practical notes for casting, recycling and safety
-
Casting: choose melt superheat carefully—too low causes poor fluidity; too high increases gas pick-up and refractory wear. Inoculants and fluxes aid solidification control.
-
Recycling (scrap melting): composition varies; control of tramp elements (Cu, Sn, P) matters more than small shifts in melting point.
-
Safety: melting steel involves molten metal hazards, slag/metal splashes, and extreme radiant heat. Use proper PPE, continuous temperature monitoring and gas control.
Useful quick conversion table (°C ↔ °F)
Table 3 — Selected conversions
| °C | °F |
|---|---|
| 1,350 °C | 2,462 °F |
| 1,375 °C | 2,507 °F |
| 1,400 °C | 2,552 °F |
| 1,425 °C | 2,597 °F |
| 1,450 °C | 2,642 °F |
| 1,475 °C | 2,687 °F |
| 1,500 °C | 2,732 °F |
| 1,525 °C | 2,777 °F |
| 1,538 °C | 2,800 °F (pure Fe) |
FAQs
-
Q: At what temperature does steel melt?
A: It depends on grade—common ranges are ≈1,370–1,530 °C (2,500–2,800 °F). Use the specific grade datasheet for exact solidus/liquidus values. -
Q: Does carbon increase or lower steel’s melting point?
A: Carbon changes the Fe–C phase relations: it can both lower parts of the melting behaviour and broaden the melting range depending on concentration and nearby phases. -
Q: Is stainless steel higher melting than carbon steel?
A: Not uniformly—many stainless grades melt in a similar window to carbon steels, though specific grades differ (e.g., 304 ~1,400–1,450 °C, 316 slightly lower). -
Q: Why are ranges (solidus–liquidus) important for casting?
A: The range defines the mushy zone where feeding and shrinkage porosity occur—designing risers and gating requires knowing it. -
Q: Can you melt steel in a backyard forge?
A: Achieving and safely handling fully molten steel requires industrial furnaces and strict safety systems—backyard forges are not appropriate for full melting operations. -
Q: How do impurities like sulfur or phosphorus affect melting?
A: They tend to form low-melting compounds or embrittling phases and can lower local melting temperatures, sometimes causing hot-shortness. -
Q: Do measurement heating rates change observed melting?
A: Yes—faster heating can shift observed onset temperatures; standardised rates give reproducible lab values. -
Q: Is the melting point the same as the maximum service temperature?
A: No — service temperatures are much lower because material strength, oxidation resistance and creep limit usable temperatures well below melting. -
Q: Which furnace is best for melting alloy steels?
A: Electric arc and induction furnaces are commonly used; the choice depends on scale, energy, and control needs. -
Q: Where to find exact solidus/liquidus for a custom alloy?
A: Use thermodynamic software (CALPHAD), consult producer datasheets, or measure via DSC/DTA in a lab with controlled atmosphere.
Final summary
Steel’s “melting point” is a practical interval driven by alloy chemistry and phase equilibria. Use grade-specific data and quality-controlled measurement when precise temperatures matter. For everyday engineering work, remember the typical band 1,370–1,530 °C, consult supplier datasheets for exact solidus/liquidus, and design processes (melting, casting, welding) around material behaviour, not a single temperature.
Authoritative references
- NIST Chemistry WebBook — Iron (Fe) reference data (melting point data)
- ASM Handbook — Chapter on Melting (Steel Castings Handbook / ASM International)
- British Stainless Steel Association — Melting temperature range for stainless steels
- PubChem / NCBI — Iron (Fe) compound summary (melting point and reference data)

