The melting point of pure elemental aluminium (Al, 99.99% or technical grade) is 660.32 °C (933.47 K; 1,220.58 °F). In practice, commercial aluminium and engineering alloys show lower or broader melting ranges: typical cast alloys begin to melt (solidus) from roughly 500–620 °C and finish melting (liquidus) up to 640–660+ °C, depending on composition.
Basic definition: melting point vs. solidus & liquidus
“Melting point” usually refers to the equilibrium fusion temperature of a pure crystalline substance, where the solid and liquid phases coexist. For pure aluminium this is a single, sharply defined temperature. For alloys, melting occurs over a range: the solidus is the temperature where melting begins (first liquid appears), and the liquidus is where melting completes (last solid disappears). In engineering practice, both endpoints matter because processing windows and microstructures depend on how much liquid is present at a given temperature.
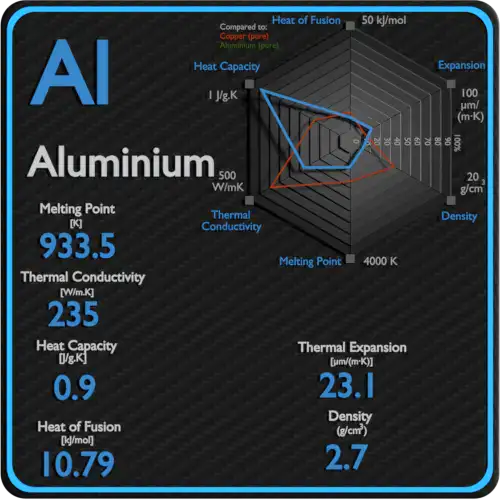
Standard accepted value for pure aluminium
High-quality sources agree on the accepted fusion temperature of elemental aluminium:
-
NIST/Chemistry WebBook and thermochemical databases report the fusion point as ~933.47 K = 660.32 °C (1,220.58 °F). This is the value used in thermophysical tables.
-
Reference compendia (CRC Handbook, RSC periodic table, Britannica) present essentially the same figure (660.3 °C), sometimes rounded to 660 °C.
These authoritative values are based on high-precision calorimetric and melting-point measurements and are the baseline for materials data sheets.
Thermodynamic and crystallographic background
Aluminium has a face-centered cubic (fcc) crystal lattice (α-Al), stable up to the fusion point. Melting requires breaking the long-range order of the lattice: the phonon and bond-energy balance is described thermodynamically by the free-energy difference between liquid and solid. The heat of fusion for aluminium is modest (≈ 10.7 kJ/mol), which matches the relatively low melting temperature compared with refractory metals. Because the fcc lattice offers many slip systems, solid aluminium is ductile up to near-melting conditions.
How alloying elements and impurities change melting behaviour
Alloying elements affect the melting interval by altering thermodynamic equilibria:
-
Melting-point depression: Many alloying elements (Si, Cu, Mg, Zn, Fe) lower the solidus or create a melting-range because they dissolve into the Al lattice or form low-melting eutectics.
-
Eutectic systems: Some binary or multi-component Al alloys have eutectic reactions producing localized liquid at temperatures significantly below 660 °C. For example, Al–Si cast alloys show eutectic melting around 577 °C depending on Si fraction.
-
High-purity vs technical grades: Ultra-high-purity Al (99.999%) shows the sharpest melting plateau; commercial grades with trace impurities may show modest melting range broadening.
-
Segregation and microstructure: During solidification, solute partitioning causes microsegregation that affects local melting behaviour during reheat or soldering.
Engineering implication: Always use alloy-specific solidus/liquidus data (not the elemental 660.32 °C) when setting furnace or welding temperatures.
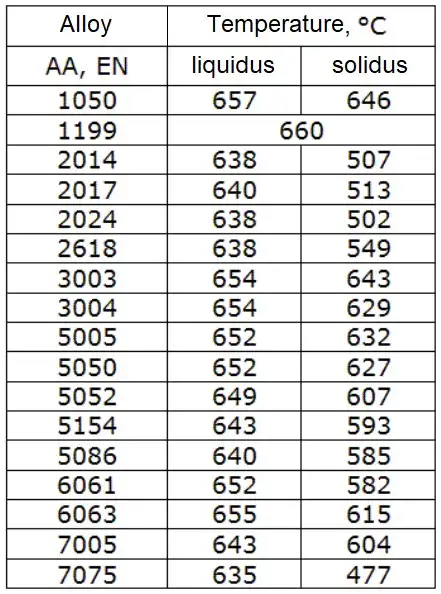
Solidus & liquidus: selected alloys (tables)
Pure aluminium (reference)
| Property | Value |
|---|---|
| Melting point (Tfus) | 660.32 °C |
| Kelvin | 933.47 K |
| Fahrenheit | 1,220.58 °F |
| Heat of fusion (ΔHf) | ≈ 10.67 kJ/mol |
| Density (solid near RT) | 2.70 g·cm⁻³ |
Representative solidus/liquidus for common Al alloys (typical ranges)
Note: alloy ranges vary with composition, temp. control, measurement method and standards (see alloy datasheets).
| Alloy family | Typical solidus (°C) | Typical liquidus (°C) | Comment |
|---|---|---|---|
| 1xxx (pure Al) | 660 | 660 | Essentially single point |
| 2xxx (Al–Cu series, e.g., 2024) | 502–505 | 635–640 | Wide melting interval due to intermetallics |
| 3xxx (Al–Mn, e.g., 3003) | ≈ 640 | ≈ 650 | Narrower range, work-hardenable |
| 4xxx (Al–Si cast/rod, e.g., 4032) | 577 | 615 | Al–Si eutectics lower melting |
| 5xxx (Al–Mg, e.g., 5083) | ≈ 570–620 | ≈ 625–640 | Mg content affects range |
| 6xxx (Al–Mg–Si, e.g., 6061) | ≈ 555–640 | ≈ 640–650 | Typical extrusion alloys |
| 7xxx (Al–Zn, e.g., 7075) | ≈ 477–610 | ≈ 608–635 | Can have low-melting precipitates |
| Cast alloys (Al–Si) | 520–570 | 575–640 | Good casting fluidity at lower temps |
(Ranges above are illustrative; consult alloy supplier datasheets or ASM/Aluminum Association sheets for precise solidus/liquidus.)
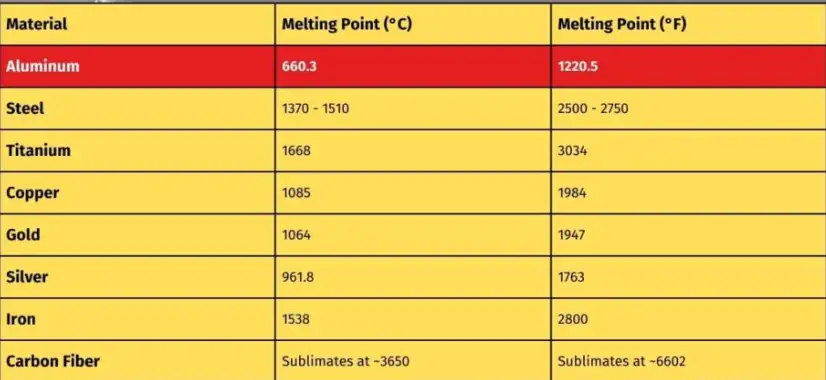
Practical consequences for melting, casting, and welding
-
Foundry practice: For melting and holding, foundries typically keep furnace temperatures 50–150 °C above the alloy liquidus to ensure fluidity, degassing and superheat required for pouring. For pure aluminium this could mean holding melts at ~720–800 °C depending on casting method and time.
-
Welding/brazing: Localized heating (e.g., TIG, MIG) must avoid exceeding the solidus of alloys near joints; preheating or interpass temps are chosen to prevent melting of heat-affected zones. Soldering and brazing use filler metals with lower melting points.
-
Heat treatment: Solution treatments are well below melt temperatures but must be chosen so the microstructure dissolves strengthening phases without approaching incipient melting (which occurs near solidus where low-melting constituents melt).
-
Recycling: Scrap mixes cast and wrought grades; melting behavior is dominated by the lowest-melting constituents and presence of contaminants (e.g., Zn, Pb), so process control and fluxing are essential.
Measurement methods, calibration and uncertainty
Common measurement techniques:
-
Differential Scanning Calorimetry (DSC): Measures heat flow; provides precise onset (solidus) and peak (fusion) temperatures for small samples. Calibration standards (e.g., indium, zinc) are required to maintain traceable accuracy.
-
Differential Thermal Analysis (DTA): Similar to DSC; cheaper but with larger uncertainty.
-
Pyrometry / optical methods: Used at foundry scale to monitor bath temperatures; emissivity and calibration are critical, and pyrometers measure surface temperature, not internal equilibrium.
-
Melting point apparatus / capillary tube: Traditional lab methods for pure substances.
Uncertainty sources: sample purity, heating rate (higher rates shift observed onset temperatures), atmosphere (oxidation raises apparent temperatures by insulating), and instrument calibration. For high-precision needs, use standard reference materials and report measurement method and heating rate.
Phase diagrams and eutectic phenomena
Phase diagrams (binary and multicomponent) show which phases are stable at given compositions and temperatures. Key features for aluminium systems:
-
Al–Si binary: A common casting system with a eutectic around ~12.6 wt% Si; eutectic temperature is significantly lower than pure Al (≈ 577 °C for the Al–Si eutectic). This is why Al–Si cast alloys are chosen for low pouring temperatures and good fluidity.
-
Al–Cu, Al–Mg, Al–Zn: These systems display intermetallics and complex primary phases that give strengthening but also create melting-range complexity.
-
Ternary interactions: Real engineering alloys are multicomponent; phase diagrams are used with computational thermodynamics (CALPHAD) to predict liquidus/solidus and precipitate formation.
Heat of fusion, density change and thermophysical properties at melting
Important numbers for process modelling:
-
Heat of fusion ΔHf: ≈ 10.67 kJ/mol (≈ 397 J/g).
-
Density change: Solid aluminium density at RT ~2.70 g·cm⁻³; density decreases on melting — typical liquid density near melting ≈ 2.37 g·cm⁻³. This contraction/expansion matters for casting shrinkage calculations.
-
Specific heat & thermal conductivity: Both increase near melting; thermal conductivity decreases sharply when solid becomes liquid. Accurate models for solidification require temperature-dependent properties.
Safety, oxidation and furnace atmosphere control
Aluminium oxidizes readily; the oxide layer (Al₂O₃) forms almost instantly and increases surface melting requirements for clean metal flow. Practical measures:
-
Fluxing & dross control: Fluxes and skimming remove oxides and impurities; dross contains entrained oxides and must be handled safely.
-
Inert or reducing atmospheres: For laboratory melting and specialized alloys, inert gas (argon) or reducing atmospheres limit oxidation. For bulk foundry operations, fluxing and good furnace designs are standard.
-
Contamination avoidance: Avoid contact with water near molten metal (steam explosions risk). Slag and contaminants with low melting points (e.g., contamination by zinc or lead) can cause hazardous splashing.
Typical industrial setpoints and control strategies
Foundry and smelting practice is performance-driven:
-
Melting furnace setpoint for general casting alloys: commonly 700–760 °C, depending on alloy and desired superheat.
-
Holding temperatures: depend on time in ladle and exposure; maintain enough superheat for metal transfer but minimize oxidation and grain growth.
-
Continuous monitoring: thermocouples (type B, S) and optical pyrometers are used with regular calibration. For critical alloys, sample analysis confirms composition before casting.
Measurement example (how to report melting data for a new alloy)
When reporting melting behavior of a laboratory-cast alloy, include:
-
Alloy chemical composition (wt% of major elements)
-
Measurement method (DSC/DTA/pyrometer) and heating rate
-
Observed solidus and liquidus with uncertainty (e.g., Solidus = 578 ± 2 °C, Liquidus = 613 ± 2 °C)
-
Sample preparation and atmosphere (argon, air)
-
Microstructural notes (eutectic phases, primary crystals)
-
Reference to calibration standards used
FAQs
1. What is the melting point of pure aluminium?
660.32 °C (933.47 K; 1,220.58 °F) is the accepted fusion temperature for elemental aluminium.
2 Why do aluminium alloys melt at different temperatures?
Alloying elements and impurities alter phase equilibria, create eutectics, and produce multi-phase microstructures that melt over a temperature range (solidus → liquidus).
3. What is the difference between solidus and liquidus?
The solidus is the temperature where melting starts; the liquidus is where melting completes. Between them the material is a mixture of solid + liquid.
4. How do I choose a furnace temperature for melting an Al alloy?
Select a temperature above the alloy’s liquidus by an appropriate superheat (typically 50–150 °C) to ensure fluidity while minimizing oxidation and gas pickup.
5. Do impurities raise or lower aluminium’s melting point?
Most common impurities lower the effective melting temperature (melting-point depression) or create low-melting eutectics. Exceptions depend on specific phase behavior.
6. Is aluminium oxide (alumina) the same as aluminium’s melting point?
No. Al₂O₃ (alumina) melts at a much higher temperature (~2,000 °C) than metallic Al (≈ 660 °C). The oxide layer complicates melting and casting but does not change the elemental melting point.
7 How precise are melting point measurements?
Lab DSC/DTA can resolve melting onsets to ±0.5–2 °C with proper calibration; industrial pyrometry has larger uncertainties due to emissivity and surface effects.
8. Can I melt aluminium in an electric furnace at home?
Small-scale aluminium melting is possible but requires appropriate equipment (refractory crucible, fluxing, ventilation). Safety hazards (hot metal, fumes, water contact) make it risky for untrained hobbyists.
9. How does melting relate to recycling aluminium?
Recycled streams mix alloys and contamination; melting behavior is dictated by the lowest-melting constituents and contaminants (e.g., Zn, Pb), so sorting and fluxing are essential to maintain quality.
10. Where can I find authoritative solidus/liquidus data for a particular alloy?
Use alloy datasheets (Aluminum Association, ASM, MatWeb, CRC), peer-reviewed phase-diagram compilations, and CALPHAD databases for accurate alloy-specific temperature data.
Practical tables for engineers
Conversion quick-reference
| Units | Value |
|---|---|
| K | 933.47 K |
| °C | 660.32 °C |
| °F | 1,220.58 °F |
Recommended processing temps
| Operation | Alloy type | Recommended range |
|---|---|---|
| Melting (foundry) | Cast Al–Si alloys | 700–760 °C |
| Melting (foundry) | Wrought alloys (recycling blends) | 720–780 °C |
| Holding (short-term) | General | liquidus + 50–100 °C |
| Solution treatment caution | Alloys with low-melting eutectics | Keep ≤ (solidus − 10 °C) to avoid incipient melting |
Authoritative notes and best practices for documentation
-
Always quote the measurement method and calibration when publishing melting data.
-
When specifying processing temperatures in contracts or drawings, cite solidus/liquidus (not only the elemental melting point).
-
Use traceable standards and supplier certificates when alloy behavior is critical to product acceptance.
Limitations and what to watch for
-
Published melting values assume thermodynamic equilibrium; rapid heating or cooling can change observed behavior.
-
Small sample purity differences and surface oxides shift measured onset points.
-
Multicomponent systems require computational thermodynamics for accurate prediction beyond binary phase diagrams.
Closing summary
Pure aluminium melts at 660.32 °C (933.47 K; 1,220.58 °F). For engineering work, however, alloy composition, microstructure, and processing history determine the actual temperatures at which melting begins and completes. For safe casting, welding and heat-treatment practice, rely on alloy-specific solidus and liquidus values from recognized standards and suppliers, apply appropriate superheat, and control atmosphere and dross formation.

