Aluminum is a metal. specifically a lightweight post-transition metal with metallic bonding, good electrical and thermal conductivity, ductility, and a characteristic face-centered cubic crystal structure. In everyday use aluminum behaves like a metal (conducts electricity, deforms plastically, forms alloys) even though some chemical properties (strong oxide film, moderate electronegativity) give it borderline behavior in certain reactions.
What chemists mean by “metal” and “nonmetal”
In chemistry and materials science, “metal” usually refers to elements or alloys that share certain physical and electronic characteristics: metallic bonding, delocalized electrons, electrical and thermal conductivity, ductility, malleability, and metallic luster. “Nonmetal” describes elements that lack delocalized metallic electrons, and typically are poor conductors and brittle in the solid state (when solids). These definitions are broad; some elements fall in between due to intermediate properties. Aluminum displays the characteristic metallic attributes listed above, so it is classified as a metal (post-transition metal).
Where aluminum sits on the periodic table
-
Element: Aluminum (United States spelling “aluminum”, IUPAC name “aluminium”)
-
Symbol: Al
-
Atomic number: 13
-
Group: Group 13 (boron group) — often described as a post-transition metal rather than a transition metal.
The "post-transition metal" tag signals that aluminum shares many metallic characteristics (metallic bonding, crystalline metal lattice) but differs from heavier Group-13 members in some bonding tendencies and reactivity.
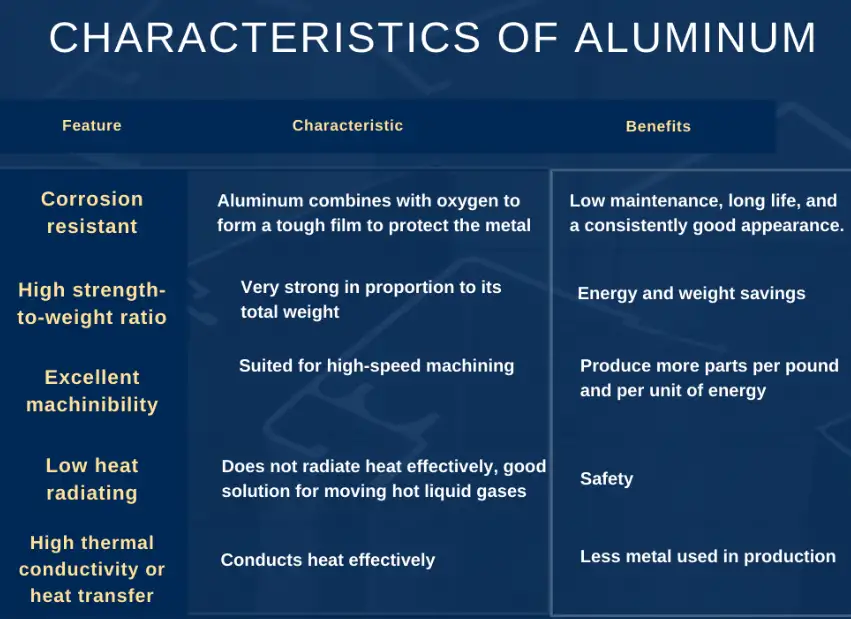
Physical properties that show aluminum is a metal
Below is a short facts table followed by a summary of why those facts point to metallic character.
Quick facts — aluminum (standard conditions)
| Property | Value |
|---|---|
| Density | ≈ 2.70 g·cm⁻³. |
| Melting point | 660.3 °C (1,220 °F). |
| Boiling point | ≈ 2,467 °C. |
| Crystal structure | Face-centered cubic (FCC). |
| Electrical conductivity | Good conductor; used widely in power transmission. |
| Thermal conductivity | High for a metal (used in heat sinks). |
| Typical oxidation state | +3 (Al³⁺). |
| Sources: NIST, Britannica, RSC. |
Why these facts imply “metal”:
-
FCC crystal structure and metallic bonding imply delocalized electrons and plasticity — typical metal behavior.
-
Electrical and thermal conductivity are hallmarks of metals; aluminum is widely used in electrical transmission for that reason.
Chemical behavior and the oxide paradox
Aluminum is highly reactive chemically — it readily forms a thin layer of aluminum oxide (Al₂O₃) on exposure to air. That oxide layer is extremely adherent and protective, producing excellent corrosion resistance in many environments. The oxide film gives aluminum a paradoxical impression: it behaves like a reactive metal by oxidizing rapidly, but the oxide then protects it, making it appear inert. This surface behavior explains many real-world observations (why household aluminum pans and building facades last a long time).
Chemically, aluminum tends to form Al³⁺ compounds (ionic/covalent character depending on partner), which fits its position in Group 13. Despite these ionic tendencies, the metallic bulk retains metallic bonding and properties.
Bonding and crystal structure explained for engineers
-
Electron configuration: [Ne] 3s² 3p¹. Aluminum has three valence electrons that can delocalize, enabling metallic bonding across the lattice.
-
Crystal lattice: Face-centered cubic (FCC). FCC gives good slip systems and high ductility; this is why aluminum and many aluminum alloys are easily formed and drawn.
Engineering takeaway: If you need a formable, lightweight conductor that can be extruded, stamped, or drawn, aluminum is a metal that performs these tasks efficiently.
Comparison table: aluminum vs typical nonmetals
| Property | Aluminum (metal) | Silicon (metalloid) | Oxygen (nonmetal) |
|---|---|---|---|
| Conductivity (solid) | Good electrical conductor | Poor conductor (semiconductor behavior) | Poor (gas) |
| Ductility | High | Brittle (when crystalline) | Gas at STP |
| Typical bonding | Metallic (delocalized electrons) | Mixed covalent/metallic | Covalent (molecules) |
| Crystal structure (solid) | FCC metal lattice | Diamond cubic (Si) | — |
| Reactivity | Forms protective oxide film | Forms oxides with more covalent character | Highly reactive—forms many oxides |
This quick comparison clarifies why aluminum fits the metal column and differs from nonmetal elements. (Data summarized from RSC, NIST, Britannica.)
Common aluminum alloys and industrial uses
Aluminum rarely appears in industry as the pure element; alloys provide strength and tailored properties.
| Alloy series | Typical composition/notes | Common uses |
|---|---|---|
| 1xxx (pure Al) | >99% Al — excellent corrosion resistance, conductivity | Chemical equipment, electrical conductors |
| 2xxx (Al-Cu) | Strengthened by copper; heat treatable | Aerospace structural components |
| 3xxx (Al-Mn) | Good formability and corrosion resistance | Beverage cans, cooking utensils |
| 5xxx (Al-Mg) | Good weldability and corrosion resistance | Ship hulls, pressure vessels |
| 6xxx (Al-Mg-Si) | Good extrudability; heat treatable | Architectural profiles, automotive parts |
| 7xxx (Al-Zn) | High strength; used in aerospace | High-strength structural components |
(Information adapted from industry sources and ASM materials guides.)
Corrosion, surface finishing, and practical durability
-
Anodizing is a controlled oxide thickening process that increases wear resistance and allows dyeing. Widely used on architectural and consumer parts.
-
Powder coating and organic paints are common for decorative and protective coatings.
-
Galvanic considerations: Aluminum is anodic relative to many steels and noble metals; designers must avoid direct contact in corrosive environments without insulation.
These are critical supplier negotiation points: ask for finish (mill, anodized Class I/II/III), required salt-spray or surface roughness specs, and acceptance criteria.
How aluminum is produced, recycled, and why that matters to buyers
-
Primary production: Bauxite → alumina (Bayer process) → aluminum (Hall-Héroult electrolysis). Production is energy-intensive; location of smelters often depends on access to cheap electricity.
-
Recycling: Secondary aluminum uses ≈5% of the energy required for primary metal. Recycled aluminum retains properties and is widely used in automotive, packaging, and construction. For cost and sustainability, many buyers insist on recycled content or specific scrap sources.
Buyer tip: Specifying recycled content and required certification (e.g., mill certificate with chemical analysis, origin statement) can protect pricing and sustainability goals.
Procurement checklist
-
Alloy series & specific temper (e.g., 6061-T6, 5052-H32)
-
Form (sheet, plate, extruded profile, billet)
-
Dimensions & tolerances (thickness, flatness, straightness)
-
Surface finish (mill finish, anodized, mill polish)
-
Mechanical requirements (tensile, yield) and acceptance tests (UT, PMI)
-
Certifications (mill test reports, ISO, REACH / RoHS if required)
-
Packaging and handling (to avoid contact with dissimilar metals)
Case 1: buyer selecting sheet aluminum
Buyer: “We need 2.0 mm sheet for a heat exchanger cover, excellent formability, and good corrosion resistance in coastal environments. Any recommendations?”
Supplier: “5052-H32 and 5754 are both good candidates — magnesium gives good marine corrosion resistance; if you need higher strength consider 6061 but note it’s less formable and requires heat treatment to reach T6.”
Case 2: buyer concerned about electrical conductors
Buyer: “We need conductors for overhead lines. Weight is critical and cost must be low.”
Supplier: “High-purity 1350 and 1370 series aluminum are standard for electrical conductors; their conductivity is good and they’re lightweight. Consider AA-1350 or 1370 with required temper.”
Case 3: buyer asking for recycled content
Buyer: “Can you supply 50% recycled content 6061 plate? We need mill certs proving chemistry and mechanicals.”
Supplier: “Yes — we can supply secondary ingots to your spec. We’ll include a mill test report (MTR) showing batch chemistry and heat number traceability.”
Practical note: Always request MTRs and acceptance criteria before release of order.
Standards and testing references
When specifying or buying aluminum, request or include the relevant standards and testing clauses in your PO:
-
ASTM standards (common examples): ASTM B209 (aluminum and aluminum-alloy sheet and plate), ASTM B221 (extruded bars, rods, profiles), ASTM B209M (metric), ASTM B456 (anodic oxide coatings).
-
ISO standards: ISO 6361 for rolled aluminum and aluminum alloys, etc.
-
Mechanical testing: tensile test per ASTM E8 / EN ISO 6892; hardness per ASTM E18 or equivalent.
-
Chemical analysis: request MTR with percent composition, traceability to lot/heat.
Including explicit standard numbers in purchase orders reduces ambiguity and supplier pushback.
FAQs
-
Is aluminum a metal or nonmetal?
Aluminum is a metal. a lightweight post-transition metal (Group 13) with metallic bonding and characteristic metal properties. -
Why does aluminum form a protective oxide if it is reactive?
Aluminum rapidly forms a thin Al₂O₃ film that adheres tightly, preventing further corrosion and making the metal effectively passive under many conditions. -
Is aluminum magnetic?
No, aluminum is nonmagnetic in its common metallic form; it is paramagnetic (very weakly attracted in strong magnetic fields). -
Can aluminum conduct electricity like copper?
Yes; aluminum conducts electricity well (≈61% of copper’s conductivity by cross-section), and its low weight makes it common in power distribution. -
What aluminum alloy is best for structural parts?
Alloys in the 2xxx and 7xxx series (Al-Cu, Al-Zn) are used for high-strength structural parts; 6xxx series balances strength and formability. -
Is recycled aluminum as good as primary aluminum?
Recycled (secondary) aluminum retains key properties and is widely used; quality depends on scrap sorting and remelting practice. -
How do I specify finish to avoid corrosion?
Specify anodizing class or organic coating with relevant salt-spray acceptance criteria; specify isolation from dissimilar metals when needed. (See ASTM standards.) -
Does aluminum rust?
“Rust” refers to iron oxides. Aluminum does not rust but does oxidize; the resulting oxide layer protects the metal. -
Is aluminum food-safe?
Many aluminum alloys and finishes are safe for food contact; check regulatory standards and finish treatments (anodized vs bare). -
What tests should I request from a mill?
Request chemical composition MTR, tensile test results, hardness, and any non-destructive tests required (UT, visual), per ASTM/ISO standards in your spec.
Practical seller/buyer checklist
-
Confirm alloy and temper.
-
Include exact dimensions and tolerances.
-
Specify required surface finish and acceptance criteria.
-
Request MTR and batch traceability.
-
Define packaging and delivery conditions.
-
Add test and inspection clauses (random sampling, destructive testing allowances).
-
Confirm environmental or recycled content requirements.
Final technical remarks
Although aluminum is sometimes described in popular writing as “borderline” due to its chemical behavior (strong oxide formation, intermediate electronegativity), its bulk physical properties and crystalline metallic structure make it clearly a metal by both chemical and engineering definitions. When specifying or discussing aluminum in procurement and design, treat it as a metal: ask for metal test certificates, tensile/yield values, and surface treatments that are standard for metallic components.

