Nickel is a hard, silver-white transition metal (symbol Ni, atomic number 28) whose high strength, corrosion resistance, and thermal stability make it indispensable in stainless steels, specialty alloys, battery cathodes, electroplating, and chemical catalysts. Strong demand from stainless steel and battery industries, major supply shifts in Indonesia and the Pacific, and high recyclability have positioned nickel at the center of modern materials and energy transitions.
1. Quick summary of nickel’s core properties
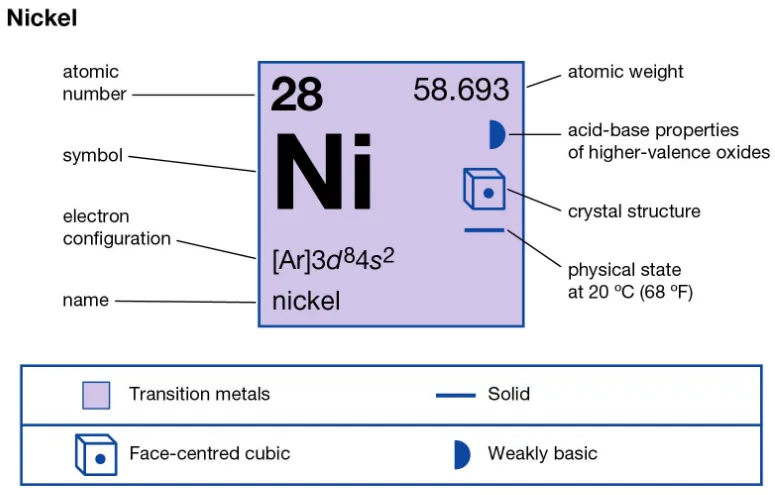
Nickel is a transition metal with atomic number 28 and standard atomic weight near 58.69. It is ductile, ferromagnetic at room temperature, and forms a protective oxide surface that slows corrosion in air. Melting point is roughly 1455 °C and density near 8.90 g/cm³. These baseline facts underpin why nickel is used in high-temperature alloys and corrosion-resistant steels.

2. Ten concise, interesting facts about Nickel
-
Nickel has atomic number 28 and strong ferromagnetism at ordinary temperatures; its Curie temperature is about 355 °C.
-
Stainless steel consumes the largest share of nickel demand; adding nickel improves toughness and corrosion resistance.
-
A single country swing can reshape supply: Indonesia’s rapid growth in laterite ore output has shifted global flows in the past decade.
-
Nickel appears in multiple refined forms: pure nickel, ferronickel, nickel matte, nickel sulfate, and nickel powder for additive manufacturing.
-
Nickel-bearing battery cathodes (NMC, NCA) trade off energy density against cost and supply security.
-
Over 60% of nickel in use is recoverable via recycling, giving nickel among the highest metal circularity benefits.
-
Nickel has both sulfide and laterite ore sources; metallurgical processing differs greatly between them.
-
Many nickel alloys keep mechanical strength at temperatures that would soften plain steels, enabling high-temperature service.
-
Nickel has stable isotopes used in nuclear and research contexts; Ni-58 and Ni-60 are among the most abundant.
-
Nickel plating remains a common surface treatment for wear and corrosion resistance in electronics, hardware, and automotive parts.
3. Physical and chemical properties (table)
| Property | Value (typical) |
|---|---|
| Symbol | Ni |
| Atomic number | 28 |
| Standard atomic weight | 58.693 |
| Density (20 °C) | 8.90 g/cm³ |
| Melting point | ≈ 1455 °C |
| Boiling point | ≈ 2913 °C |
| Crystal structure | Face-centered cubic |
| Common oxidation states | +2, (some +3 compounds) |
| Curie temperature | ≈ 355 °C |
| (Data sources: Royal Society of Chemistry and Nickel Institute.) |
4. Where nickel comes from: ores, mining, and global production
Nickel occurs in two main deposit types with very different metallurgy:
-
Sulfide deposits: typically lower-tonnage but higher-grade, easier to concentrate and smelt into matte. Classic producers include parts of Canada, Russia, and Australia.
-
Laterite deposits: weathered, near-surface ores abundant in tropical zones. Laterites dominate recent mine growth in Indonesia, the Philippines, and New Caledonia. Processing laterite into battery-grade chemicals requires different hydrometallurgical routes.
Top producing countries (recent trend snapshot)
| Country | Notes |
|---|---|
| Indonesia | Massive recent expansion in laterite mining and ore exports; drives global supply. |
| Philippines | Significant laterite output with evolving domestic processing. |
| Russia | Historic sulfide production and integrated refining. |
| Canada | Sulfide mines, higher-grade concentrates exported for smelting. |
| Australia | Mixed mine types, growing refined output. |
Authoritative production data and year-on-year shifts are reported annually by national geological services and the USGS. For example, recent USGS commodity summaries provide production, trade, and consumption figures that buyers and analysts use to model supply risk.
5. How nickel is processed and refined into market forms
Raw ore moves through several stages before becoming a commercial product:
-
Mining and crushing
-
Concentration (for sulfides) or direct drying/smelting (for some laterites)
-
Smelting to produce nickel matte or ferronickel
-
Refining and chemical conversion to produce nickel metal, nickel sulfate, or other chemicals for battery use
-
Casting, powder atomization, or electroplating to produce final shapes and coatings
Different refining routes yield different downstream use suitability. For example, high-purity nickel sulfate is the feedstock for many lithium-ion battery cathode manufacturers, while ferronickel is suited for stainless steel production. USGS and industry factbooks map these routes and trade flows.
6. Principal applications and why nickel is chosen
Nickel’s mechanical and chemical features deliver specific performance benefits:
-
Stainless steel: Nickel increases toughness, ductility, and resistance to corrosion and oxidation. Grades such as 304 and 316 stainless contain nickel to stabilize the austenitic structure and improve service life.
-
High-performance alloys: Nickel-based superalloys retain strength at elevated temperatures, critical in turbines, aerospace, and chemical processing.
-
Batteries: Nickel compounds are central to cathode chemistries that target higher energy density.
-
Electroplating and coatings: Nickel provides a smooth, corrosion-resistant surface and is often used beneath chrome or other finishes.
-
Catalysts and chemical production: Nickel catalysts enable hydrogenation and hydrogen reforming reactions.
-
Additive manufacturing and powders: Nickel powders are used in 3D printing alloys where a balance of strength and corrosion resistance is necessary.
Each application demands specific impurity limits and form factors, which is why nickel’s supply chain distinguishes between battery-grade chemicals, metallurgical feedstocks, and high-purity metal.
7. Nickel in batteries and the energy transition
Nickel’s role in lithium-ion battery cathodes has grown because higher nickel content raises energy density and lowers reliance on cobalt. Systems labeled NMC or NCA reference nickel-manganese-cobalt or nickel-cobalt-aluminum blends. That means successful integration of nickel into the energy system depends on producing battery-grade nickel sulfate and maintaining supply chain transparency to meet ethical and environmental standards. Industry groups and analysts track shifts in mining, refining, and electrochemical demand to forecast future pricing and investment needs.

8. Environmental, health, and recycling considerations
-
Recycling: Nickel is highly recyclable and retains value in scrap; stainless steel recycling loops recover large volumes, which reduces primary mining needs. Nickel Institute materials and industry programs document high recovery rates and best practices for end-of-life management.
-
Environmental risks: Laterite mining can affect tropical landscapes and water systems; proper permitting, tailings management, and progressive rehabilitation are critical. Policy changes in producing countries influence project economics and permit timelines.
-
Health and safety: Nickel compounds, particularly soluble salts and certain dusts, can be sensitizers and carcinogenic in specific forms and exposures. Workplace controls, proper PPE, and regulatory compliance keep operations within safe limits. Public-health guidance and regulatory thresholds are available from occupational agencies and material safety datasheets.
9. Common nickel alloys and when to choose each (comparison table)
| Alloy / Product | Typical composition notes | Typical uses |
|---|---|---|
| Pure nickel (Ni 200, Ni 201) | High-purity nickel with low C content | Chemical equipment, electrical resistance elements |
| Monel (e.g., Monel 400) | Nickel-copper alloy | Marine hardware, corrosion resistance in sulfide-rich media |
| Inconel (nickel-chromium superalloys) | Nickel-chromium-iron, with additions of Mo, Nb | High-temperature turbines, aerospace |
| Hastelloy (nickel-molybdenum-chromium) | Highly corrosion resistant | Chemical process plants, oxidizing/reducing environments |
| Ferronickel | Iron-nickel alloy produced from laterite | Feedstock for stainless steel production |
Each alloy family meets distinct mechanical and corrosion performance targets. Buyers choose based on operating temperature, corrosive species, mechanical loading, and fabrication method.

10. How buyers and specifiers evaluate nickel: quality, form, and price signals
Key evaluation points for purchasers and engineers:
-
Form: powder, sheet, bar, wire, plate, foil, matte, sulfate. Correct form reduces downstream processing costs.
-
Chemistry: impurity limits for sulfur, phosphorus, iron, copper, and other trace elements differ by application. Battery chemistries require tight controls on chloride and transition impurities.
-
Mechanical data: tensile strength, elongation, and creep performance for alloy choices.
-
Supply chain transparency: traceability from mine to refinery to finished product matters for procurement teams.
-
Market signals: stainless steel output, battery cell demand, and production policies in large suppliers (e.g., Indonesia) drive short-term pricing and long-term investment. Public reports from USGS and international industry groups are primary references for forecasting.
11. Frequently asked questions
Q1: Is nickel magnetic?
Nickel is ferromagnetic at room temperature, meaning it displays strong magnetic properties until heated past its Curie point around 355 °C.
Q2: Which nickel form is used in lithium-ion battery cathodes?
Battery cathodes commonly use nickel sulfate and nickel hydroxide intermediates that are processed into active cathode materials in cell manufacturing.
Q3: How does nickel content influence stainless steel?
Adding nickel stabilizes austenite, improves toughness and corrosion resistance, and helps maintain ductility at low temperatures; the specific percent depends on grade.
Q4: What is the difference between laterite and sulfide nickel ores?
Sulfides are amenable to flotation and smelting, laterites are weathered ores processed by pyrometallurgy or hydrometallurgy; each route affects final product type and cost.
Q5: Can nickel be recycled indefinitely?
Yes; nickel retains its metallurgical properties through recycling, and large proportions of in-use nickel come from recycled stainless and alloy scrap.
Q6: Are nickel compounds hazardous?
Some soluble nickel salts and dusts require strict controls due to sensitization and carcinogenicity risks; solid metal in bulk form poses much lower risk with normal handling. Follow MSDS guidance and local regulations.
Q7: How should buyers request nickel for high-temperature service?
Specify the alloy family (Inconel, Hastelloy), the required mechanical properties at operating temperature, and the acceptable heat treatment and fabrication route.
Q8: What drives short-term nickel price swings?
Inventory changes, stainless steel mill demand, battery industry announcements, and policy or export controls in producing countries are primary short-term drivers. USGS and market reports track these signals.
Q9: Is nickel used in coins and why was it named nickel?
Historically, coins used nickel alloys; the element name comes from a German mining term referring to an ore that miners thought contained copper but did not. Nickel’s coin use has declined in many jurisdictions.
Q10: How to select a supplier for industrial nickel products?
Look for manufacturers with documented quality systems, traceability from raw material to finished product, consistent chemical and mechanical testing reports, and the ability to supply the form you need at competitive terms.
12. About MWAlloys — factory pricing and customization
MWAlloys supplies nickel-containing metal products and custom alloy solutions. Our strengths: factory-direct pricing, production control, and flexible customization for chemistry and form factor. Common offerings include nickel bars, plates, powders, and bespoke alloy processing for high-performance industrial use. Contact MWAlloys for a quotation specifying required alloy grade, form, dimensions, and certification needs. Note to procurement teams: providing intended application and mechanical requirements shortens quote lead time.
Closing notes and recommended references
For technical reference and up-to-date market data, consult: the Royal Society of Chemistry element pages, the Nickel Institute materials, and USGS Mineral Commodity Summaries. These sources offer authoritative property tables, application breakdowns, and annual commodity statistics that procurement, engineering, and sustainability teams rely on.

